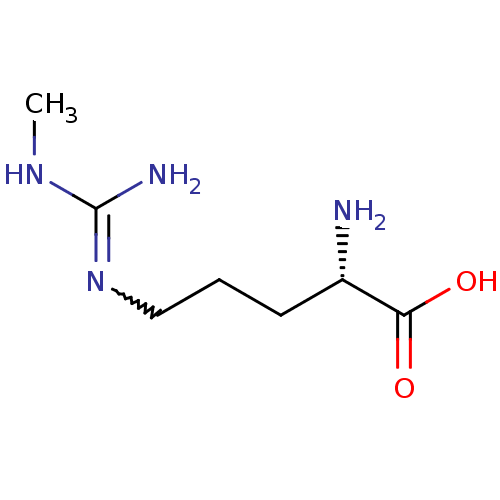
BDBM50230993 (2S)-2-amino-5-[(N-methylcarbamimidoyl)amino]pentanoic acid::(2S)-2-ammonio-5-{[iminio(methylamino)methyl]amino}pentanoate::(R)-2-Amino-5-(N'-methyl-guanidino)-pentanoic acid::(S)-2-Amino-5-(N'-methyl-guanidino)-pentanoic acid::(S)-2-amino-5-(2-methylguanidino)pentanoic acid::(S)-2-amino-5-(3-methylguanidino)pentanoic acid::2-Amino-5-(N'-methyl-guanidino)-pentanoic acid::CHEMBL256147::L-NMMA::N omega-methyl-L-arginine::N(G)-mono-methyl-L-arginine::N(G)-monomethyl-L-arginine::N-Monomethyl-L-arginine::N-gamma-monomethyl-L-arginine::NG-Monomethyl-L-Arginine::NG-monomethyl-L-arginine acetate::Ngamma-monomethyl-L-arginine::Targinine
SMILES CNC(N)=NCCC[C@H](N)C(O)=O
InChI Key InChIKey=NTNWOCRCBQPEKQ-YFKPBYRVSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50230993
Found 14 hits for monomerid = 50230993